Kistler maXYmos Provides Quality Assurance for Medical Device Manufacturers
The Kistler maXYmos TL ML is the first process monitoring system to meet the strict FDA and MDR regulatory requirements for quality assurance in the MedTech sector. The new monitoring system can deliver 100 per cent testing; a critical requirement for manufacturers operating in environments where pharmaceutical goods and MedTech equipment are produced.
The new Kistler maXYmos TL ML, based on the tried-and-tested maXYmos system, can be easily qualified and validated for integration into existing quality management systems.
Process monitoring systems play an increasingly important part in quality assurance on automated production lines. Medical device manufacturers must demonstrate that their products meet the requirements for safety and quality and provide proof of the reliability and precision of quality assurance monitoring not only throughout their own manufacturing process but also that of their suppliers.
Excluding liability – a critical objective for medical device manufacturers
In extreme cases, inadequate quality assurance, during the production of medical devices, can cause injury or even loss of life. Companies that place medical devices on the market are fully liable in the event that their products fail to function accurately and consistently. This is partly why the industry is subject to such strict regulation. Producers of medical devices, as well as plant and machinery manufacturers operating in the MedTech and pharmaceutical sectors, are confronted with huge challenges, especially when it comes to integrating process monitoring systems into automated production and packing processes.
A process monitoring system with FDA and MDR-compliant functionalities
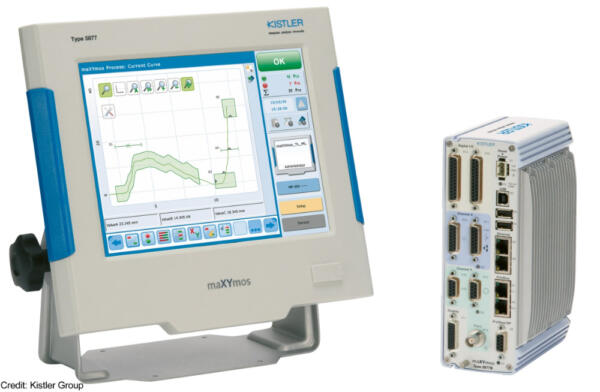
Kistler has developed this new solution based on its maXYmos TL (Top Level) well-proven system. Like all the systems in the maXYmos family, maXYmos TL visualises process profiles and offers an extensive range of interfaces for connecting sensors. The system is integrated directly into the production line to monitor and evaluate the quality of every step in the manufacturing process based on a curve. With the help of evaluation objects (EOs), users can adapt the curve evaluation to the specific monitoring task. For instance, the tolerances defined in the process validation can be used for this purpose. Based on this specification, the system then decides whether each workpiece is good or bad.
The functions integrated into maXYmos TL ML comply with the regulatory requirements for applications in the MedTech industry. The system hardware also meets the specific requirements for measurement equipment that apply in the MedTech industry, including:
- Designed to accommodate exceptionally small measuring ranges (force-displacement monitoring, torque sensor technology)
- Integrated user management compliant with FDA regulatory requirements
- Audit trail of all changes to testing processes, with time and user indexing for end-to-end traceability of each individual product
- Optional blocking of ports for secure integration into the customer’s data structure
- Direct printer connection so that test records can be documented as hard copies
Optimised production processes give manufacturers a competitive edge
The new maXYmos TL ML process monitoring system from Kistler gives producers of medical devices, as well as machinery and plant manufacturers operating in the MedTech and pharmaceutical sectors, a much easier solution to the validation of their production processes. With 100 per cent inspection of each step in the manufacturing process, the need for mandatory process validation in production can be completely eliminated.
Qualification of the production equipment is the first requirement for proof of the system’s safety. For this purpose, Kistler supplies ready-to-use checklists for Installation Qualification (IQ) and Operational Qualification (OQ). An in-plant calibration can then be performed to validate the measurement system at any time – a service that Kistler offers for its customers throughout the world. This also makes requalification of assembly plants much simpler, because the entire measuring chain is calibrated. The key benefit to manufacturers is that they can bring their product developments and innovations to market much more quickly, giving them the competitive edge that is such a critical factor in this industry.
Into the future with intelligent networking and control
The maXYmos TL ML system supports OPC UA so that it can be easily connected to machine controls and communicate with higher-level control and management systems.

Kistler UK
+44 (0) 1256 741 550
Website