Plasticom and Blink Medical Produce Novocaine Syringes
In this case study, The Plasticom Group, a toolmaking and moulding business based in Kent, details the development and manufacture of high-quality, single-use novocaine syringes.
Background
Blink Medical was founded in 2004 to specialise in supplying single-use surgical instruments. Today the company has expanded to distribute products internationally, selling products to over 20 countries worldwide. The company focuses on delivering high-quality medical devices and world-class service.
The Challenge
In May 2013, the EU introduced a directive requiring medical practices to use protective needlesticks to protect patients and end-users from injury or cross-contamination. In the UK alone, there were 40,000 needlestick injuries a year, with a large number of cases unreported costing the NHS an estimated £500,000 annually. Blink Medical wanted to design a product that protected the user, made it easy to install the cartridge and provide a product that could handle the demanding sterilisation process.
The Plasticom service in action
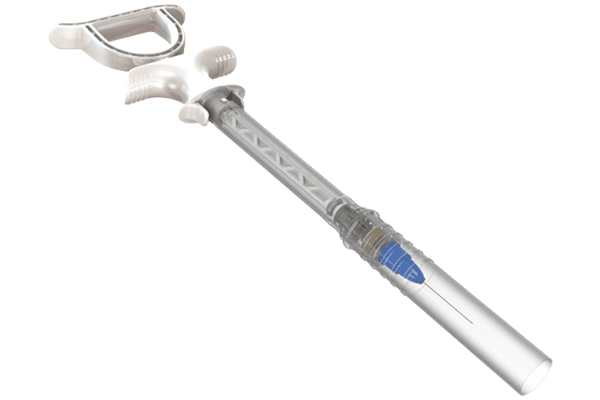
Blink Medical approached Plasticom to look at the possibility of designing a two-part syringe for the dental industry for novocaine which would meet EU requirements. Plasticom’s initial action was to look at what current products on the market offered as an answer to the EU directive. Once the Plasticom team understood what was required, they set about developing several prototypes to test the feasibility for production. A major challenge was evaluating material options to achieve a specification that would have the ability to be sterilised without losing integrity, becoming weak and compromising product quality.
Following much discussion, positive teamwork and successful prototyping, a final design was agreed with Blink Medical.
Key features include:
- A T bar handle for greater control
- Unique 0 rings to prevent cross-contamination
- Two-part barrel with a protective sleeve to ensure the needle is protected
- Cartridge installation locking mechanism with protective sleeve
- Ease of use
- Ability to be sterilised without compromising quality
Positive results
From developing the moulding tool, Plasticom is now moulding the syringes and supplying Blink Medical with a high-quality product that meets the stringent EU requirements.
Through the design and manufacturing process Plasticom has used their experience and skill to continually implement quality improvements and drive production efficiencies to improve quality and service to Blink Medical.
Plasticom is a quality plastic moulding and toolmaking business based in Ashford, Kent. They are uniquely placed to offer you first-class service.
- High quality, up to date moulding equipment
- In house toolmaking to provide fast, high quality and seamless service
- Specialist medical experience and certification to ISO 13485
- Dedicated cleanroom facility
- A problem solving, solutions and partnering approach focused on improving your business
- Just in time supply and local to you to help reduce your costs
- Unique biodegradable materials
The Plasticom Group
01233 621601
Website
Email






